The Race to a Vaccine
The Race to a Vaccine
When will this be over? How long will this last? When can the world go back to normal? Since March, these questions have been circulating in the minds of many around the world and have yet to be answered. COVID-19 has dominated the lives of people globally since the World Health Organization declared the virus to be a global pandemic on March 11, 2020. Lockdowns ensued and schools, businesses, and public places were forced to shut down. Across the globe, people were forced to quarantine which has lasted longer than originally expected.
COVID-19 stands for “coronavirus disease 2019” and is caused by the SARS-CoV-2 virus strain. It spreads through person-to-person contact – an interaction such as coughing, sneezing, or talking between infected and non-infected persons. Originally, COVID -19 was expected to fade away with the warm weather, but at this point, the only hope to return to normalcy will be a successful vaccine. Unfortunately, even as COVID cases surpass 50 million worldwide, the possibility of a vaccine coming out soon may not be very realistic.
Vaccination is the process of “Inoculating an individual with any vaccine in order to induce or increase immunity”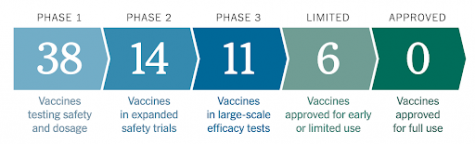 (Oxford English Dictionary). Vaccines expose cells within the body to a weakened form of a virus to stimulate an immune response. Currently, there are 52 vaccines for COVID-19 in clinical trials, and more than 86 more being tested on Animals. As of right now, the most promising vaccine candidates are the Moderna and AstraZeneca vaccines – although these two vaccines are still in development and trial. Vaccine developers and pharmaceutical companies are racing to find a COVID vaccine and many have promised a successful immunization before the end of 2021. Realistically, however, vaccines take 10 to 15 years to be perfected. A successful vaccine goes through three phases before being approved consisting of a preclinical phase, safety trials (phase 1), expanded trials (phase 2), and efficacy trials (phase 3).
(Oxford English Dictionary). Vaccines expose cells within the body to a weakened form of a virus to stimulate an immune response. Currently, there are 52 vaccines for COVID-19 in clinical trials, and more than 86 more being tested on Animals. As of right now, the most promising vaccine candidates are the Moderna and AstraZeneca vaccines – although these two vaccines are still in development and trial. Vaccine developers and pharmaceutical companies are racing to find a COVID vaccine and many have promised a successful immunization before the end of 2021. Realistically, however, vaccines take 10 to 15 years to be perfected. A successful vaccine goes through three phases before being approved consisting of a preclinical phase, safety trials (phase 1), expanded trials (phase 2), and efficacy trials (phase 3).
The AstraZeneca vaccine is being co-developed by Oxford University and is a viral vector vaccine. Viral vector vaccines are “vaccines that contain viruses engineered to carry coronavirus genes” either to “enter cells and cause them to make viral proteins” or to “slowly replicate, carrying coronavirus proteins on their surface” (The New York Times). AstraZeneca’s vaccine is based on a chimpanzee adenovirus – or a virus that can cause the common cold. During phase 1 of the vaccine trial, researchers found that the vaccine raised both coronavirus antibodies and other immune defenses. Currently, the AstraZeneca vaccine is in both phase 2 and 3 trials in order to maximize development efficiency. Phase 3 study results are expected by the end of December 2020, and planning is underway to supply hundreds of millions of doses around the world hopefully starting in January 2021.
Besides AstraZeneca, Moderna’s vaccine is another favorable candidate. The Moderna vaccine was developed by using mRNA, genetic material used to create proteins within human cells. Moderna is trying to create coronavirus proteins in order to generate an immune response that would contain a very small piece of mRNA “coding for the ‘spike protein’ of SARS-CoV-2, which targets the surface of human cells” (Bill of Health). As the ‘spike protein’ is created, the immune system should attack and destroy the ‘spike protein’. Because the ‘spike protein’ contains genetic material from the coronavirus, the immune system would learn how to fight COVID-19 by destroying it. Moderna, a biotechnology company, partnered with researchers at the National Institutes of Health in vaccine development. As of July 27, 2020, Moderna is currently in phase 3 of the vaccination trials. Phase 3 requires testing the vaccine on thousands of people to prove that the vaccine is at least 50% effective. Moderna previously found promising results in its previous trials on both monkeys and people. According to the CEO of Moderna, the company should be aware of the vaccine’s effectiveness by Thanksgiving and hopefully, it will be ready for distribution by early 2021.
As vaccine manufacturers race to find an effective immunization method, the COVID-19 pandemic has gotten much worse. As the world averages greater than 600,000 cases per day, the United States is nearing a total of 11 million cases and 300,000 deaths. Although the Moderna and AstraZeneca vaccines are promising, there are hundreds of other vaccines in development that may also prove successful. Recently released data from Pfizer – a pharmaceutical company paired with the German drugmaker BioNtech- announced that early analysis of the vaccine proved to be “more than 90 percent effective in preventing the disease among trial volunteers who had no evidence of prior coronavirus infection” (New York Times). If the remaining data collected is consistent, Pfizer plans to ask the FDA for emergency authorization later this month. Pfizer hopes to manufacture enough doses to immunize up to 20 million people by the end of 2020. Producing a vaccination is not a competition between developers, but instead a worldwide collaboration. To control COVID-19, more than one vaccine will be needed in order to vaccinate the billions of people around the world. Realistically, even if a vaccine is approved by the government before the end of 2020 or the beginning of 2021, the processes of manufacturing the vaccine and vaccinating the general public will likely take a significant amount of time. As the world waits impatiently for a vaccine to control the ravaging effects of COVID-19, individuals must also remember that other safety methods are still effective. Wearing a mask prevents the spread of COVID-19 tremendously, as does social distancing. Every individual has a social responsibility to do their part in minimizing the spread of COVID-19.
Works Cited
CDC. “How to Protect Yourself & Others.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 2020, www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
Abbasi, Jennifer. “COVID-19 and MRNA Vaccines-First Large Test for a New Approach.” JAMA, JAMA Network, 22 Sept. 2020, jamanetwork.com/journals/jama/fullarticle/2770485?resultClick=1.
Brooks, Khristopher J. “AstraZeneca Says COVID-19 Vaccine Could Arrive by Year-End.” CBS News, CBS Interactive, 5 Nov. 2020, www.cbsnews.com/news/covid-vaccine-astrazeneca-ceo-says-december-january/.
Corum, Jonathan, et al. “Coronavirus Vaccine Tracker.” The New York Times, The New York Times, 10 June 2020, www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html.
The New York Times. “New York City Covid Map and Case Count.” The New York Times, The New York Times, 9 May 2020, www.nytimes.com/interactive/2020/nyregion/new-york-city-coronavirus-cases.html?auth=login-email.
Smith, Dale. “How Soon Will There Be a COVID Vaccine? This Is Where Development Stands Now.” CNET, CNET, 29 Oct. 2020, www.cnet.com/how-to/how-soon-will-there-be-a-covid-vaccine-this-is-where-development-stands-now/.
Staff, The Petrie-Flom Center. “How Does Moderna’s COVID-19 Vaccine Work, and Who Is Funding Its Development?” Bill of Health, 25 Aug. 2020, blog.petrieflom.law.harvard.edu/2020/08/27/moderna-covid19-vaccine-government-funding/.
Thomas, Katie, et al. “Pfizer’s Early Data Shows Vaccine Is More Than 90% Effective.” The New York Times, The New York Times, 9 Nov. 2020, www.nytimes.com/2020/11/09/health/covid-vaccine-pfizer.html?searchResultPosition=1https://www.nytimes.com/2020/11/09/health/covid-vaccine-pfizer.html?searchResultPosition=1.
“Vaccination”, Oxford English Dictionary www.oed.com
Isabella is a junior at BASIS Independent Brooklyn. She loves exploring psychology and science and is passionate art and photography. Outside of school,...